Age-Related Macular Degeneration
Role of ARMS2/HTRA1 risk alleles in the pathogenesis of neovascular age‑related macular degeneration
Age‑related macular degeneration (AMD) is one of the leading causes of severe irreversible blindness worldwide in the elderly population. AMD is a multifactorial disease mainly caused by advanced age, environmental factors, and genetic variations. Genome‑wide association studies (GWAS) have strongly supported the link between ARMS2/HTRA1 locus on chromosome 10q26 and AMD development, encompassing multiple variants, rs10490924 (c.205G > T, p.A69S in ARMS2), insertion/deletion (del443/ins54 in ARMS2), and rs11200638 (in HTRA1 promoter region). In this comprehensive review, we provide an overview of the role played by ARMS2/HTRA1 risk alleles in neovascular AMD pathogenesis, covering GWAS, in vitro studies, and animal models, shedding light on their underlying molecular genetic mechanisms. Further extensive research is also imperative, including confirmation of these findings, identifying novel treatment targets, and advancing primary and secondary prevention strategies for AMD.
Pan Y, Iwata T. Role of ARMS2/HTRA1 risk alleles in the pathogenesis of neovascular age-related macular degeneration. Taiwan J Ophthalmol. 2024 Feb. DOI: 10.4103/tjo.TJO-D-23-00152
Exploring the contribution of ARMS2 and HTRA1 genetic risk factors in age-related macular degeneration
Age-related macular degeneration (AMD) is the leading cause of severe irreversible central vision loss in individuals over 65 years old. Genome-wide association studies (GWASs) have shown that the region at chromosome 10q26, where the age-related maculopathy susceptibility (ARMS2/LOC387715) and HtrA serine peptidase 1 (HTRA1) genes are located, represents one of the strongest associated loci for AMD. However, the underlying biological mechanism of this genetic association has remained elusive. In this article, we extensively review the literature by us and others regarding the ARMS2/HTRA1 risk alleles and their functional significance. We also review the literature regarding the presumed function of the ARMS2 protein and the molecular processes of the HTRA1 protein in AMD pathogenesis in vitro and in vivo, including those of transgenic mice overexpressing HtrA1/HTRA1 which developed Bruch's membrane (BM) damage, choroidal neovascularization (CNV), and polypoidal choroidal vasculopathy (PCV), similar to human AMD patients. The elucidation of the molecular mechanisms of the ARMS2 and HTRA1 susceptibility loci has begun to untangle the complex biological pathways underlying AMD pathophysiology, pointing to new testable paradigms for treatment.
Pan Y, Fu Y, Baird PN, Guymer RH, Das T, Iwata T. Prog Retin Eye Res. 2022 Dec 27:101159. doi: 10.1016/j.preteyeres.2022.101159. Online ahead of print.PMID: 36581531
Binding of Gtf2i-β/δ transcription factors to the ARMS2 gene leads to increased circulating HTRA1 in AMD patients and in vitro.
The disease initiating molecular events for Age-related macular degeneration (AMD), a multi-factorial retinal disease affecting many millions of elderly individuals worldwide, are still unknown. Of the over 30 risk and protective loci so far associated with AMD through whole genome-wide association studies (GWAS), the Age-Related Maculopathy Susceptibility 2 (ARMS2) gene locus represents one of the most highly associated risk regions for AMD. A unique insertion/deletion (in/del) sequence located immediately upstream of the High Temperature Requirement A1 (HTRA1) gene in this region confers high risk for AMD. Using electrophoretic mobility shift assay (EMSA), we identified that two Gtf2i-β/δ transcription factor isoforms bind to the cis-element 5`- ATTAATAACC-3` contained in this in/del sequence. The binding of these transcription factors leads to enhanced upregulation of transcription of the secretory serine protease HTRA1 in transfected cells and AMD patient-derived induced pluripotent stem cells (iPSCs). Overexpression of Htra1 in mice using a CAG-promoter demonstrated increased blood concentration of Htra1 protein, cause upregulation of vascular endothelial growth factor (VEGF) and produced a choroidal neovascularization (CNV)-like phenotype. Finally, a comparison of 478 AMD patients to 481 healthy, age-matched controls from Japan, India, Australia and the USA showed a statistically increased level of secreted HTRA1 blood concentration in AMD patients compared to age-matched controls. Taken together, these results suggest a common mechanism across ethnicities whereby increased systemic blood circulation of secreted serine protease HTRA1 leads to subsequent degradation of Bruch's membrane and eventual CNV in AMD.
Pan Y, Iejima D, Nakayama M, Suga A, Noda T, Kaur I, Das T, Chakrabarti S, Guymer RH, DeAngelis MM, Yamamoto M, Baird PN, Iwata T. J Biol Chem. 2021 Feb 23:100456. doi: 10.1016/j.jbc.2021.100456. Online ahead of print.PMID: 33636181
A prospective multicenter study on genome wide associations to ranibizumab treatment outcome for age-related macular degeneration
We conducted a genome-wide association study (GWAS) on the outcome of anti-VEGF treatment for exudative age-related macular degeneration (AMD) in a prospective cohort. Four hundred and sixty-one treatment-naïve AMD patients were recruited at 13 clinical centers and all patients were treated with 3 monthly injections of ranibizumab followed by pro re nata regimen treatment for one year. Genomic DNA was collected from all patients for a 2-stage GWAS on achieving dry macula after the initial treatment, the requirement for an additional treatment, and visual acuity changes during the 12-month observation period. In addition, we evaluated 9 single-nucleotide polymorphisms (SNPs) in 8 previously reported AMD-related genes for their associations with treatment outcome. The discovery stage with 256 patients evaluated 8,480,849 SNPs, but no SNPs showed genome-wide level significance in association with treatment outcomes. Although SNPs with P-values of <5 × 10-6 were evaluated in replication samples of 205 patients, no SNP was significantly associated with treatment outcomes. Among AMD-susceptibility genes, rs10490924 in ARMS2/HTRA1 was significantly associated with additional treatment requirement in the discovery stage (P = 0.0023), and pooled analysis with the replication stage further confirmed this association (P = 0.0013). ARMS2/HTRA1 polymorphism might be able to predict the frequency of injection after initial ranibizumab treatment.
Yamashiro K, Mori K, Honda S, Kano M, Yanagi Y, Obana A, Sakurada Y, Sato T, Nagai Y, Hikichi T, Kataoka Y, Hara C, Koyama Y, Koizumi H, Yoshikawa M, Miyake M, Nakata I, Tsuchihashi T, Horie-Inoue K, Matsumiya W, Ogasawara M, Obata R, Yoneyama S, Matsumoto H, Ohnaka M, Kitamei H, Sayanagi K, Ooto S, Tamura H, Oishi A, Kabasawa S, Ueyama K, Miki A, Kondo N, Bessho H, Saito M, Takahashi H, Tan X, Azuma K, Kikushima W, Mukai R, Ohira A, Gomi F, Miyata K, Takahashi K, Kishi S, Iijima H, Sekiryu T, Iida T, Awata T, Inoue S, Yamada R, Matsuda F, Tsujikawa A, Negi A, Yoneya S, Iwata T, Yoshimura N.
Sci Rep. 2017 Aug 23;7(1):9196. doi: 10.1038/s41598-017-09632-0. PMID: 28835685
HTRA1 Overexpression Induces the Exudative Form of Age-related Macular Degeneration
Age-related macular degeneration (AMD) is a leading cause of vision loss and blindness in the elderly. The dry form is more common and accounts for about 85-90% of AMD patients in US, while Japanese AMD patients predominantly progress to wet-form or polypoidal choroidal vasculopathy (PCV). Recent studies have shown HTRA1, a serine protease gene, as major risk factor for wet form AMD (De Wan et al., Science 2006). Furthermore, we reported that the Japanese typical wet form AMD patients showed significant association with ARMS2/HTRA1 (Goto, Akahori et al., JOBDI 2009). The purpose of this study is to elucidate the function of ARMS2/HTRA1 gene promoter in wet-form AMD patients. The promoter sequence experiment showed that a great number of AMD patients had specific indel mutation in 3.8 kb upstream of HTRA1 gene. 2-3-fold increase of promoter activity was observed in indel HTRA1 promoter compared to control sequence (Iejima et al., JBC 2015). Furthermore, we created transgenic mice ubiquitously overexpressing mouse HtrA1 using the chicken act in promoter, continuous induction of HtrA1 in vivo was shown to lead to CNV, similar to wet AMD patients (Nakayama, Iejima et al., IOVS 2014). These results suggest that human HTRA1 expression is enhanced by AMD specific indel mutation in the promoter region of HTRA1 gene, and this enhanced HTRA1 may be concerned with induce retinal neovasucularization.
Iejima D, Nakayama M, Iwata T. J Stem Cells. 2015;10(3):193-203.
Overexpression of HtrA1 and exposure to mainstream cigarette smoke leads to choroidal neovascularization and subretinal deposits in aged mice
We determined the function of ARMS2 and HtrA1 in the choroid and retina using transgenic (Tg) mice and evaluated the effects of mainstream cigarette smoke on these mice. The chicken actin promoter (CAG) was used to drive mouse HtrA1, human ARMS2, and ARMS2 (A69S) expression in the entire body of a mouse for one year. Fundus observations were performed with a Spectralis HRA+ optical coherence tomograph (OCT). Eyes were sectioned, stained with hematoxylin and eosin (H&E), and analyzed with immunohistochemistry. Mice were exposed to cigarette smoke for 30 min/d, 5 d/wk for 12 weeks using a mainstream smoking chamber (INH06-CIGR02A, MIPS). After 12 weeks, fundus observations and pathological analyses were performed. Approximately 18.2% of 12-month-old HtrA1 Tg mice exhibited choroidal neovascularization (CNV) by OCT and positive immunostaining with anti-CD31 and anti-fibronectin antibodies. Furthermore, elastic van Gieson (EVG) staining showed Bruch's membrane damage in HtrA1 Tg mice. No retinal changes were observed in ARMS2 and ARMS2 (A69S) Tg mice. A total of 12 weeks of exposure to mainstream cigarette smoke led to CNV rates of 7.7% for wild type (Wt) mice and 20% for HtrA1 Tg mice, but had no effect on ARMS2 Tg mice. In addition, abnormal deposits were observed between photoreceptor cells and the RPE in an HtrA1 Tg mouse exposed to mainstream cigarette smoke. The HtrA1 overexpression and mainstream cigarette smoke can independently lead to CNV. The HtrA1 gene is a strong risk factor for wet AMD, but not all of the HtrA1 Tg mice developed CNV, suggesting that CNV development depends on multiple risk factors.
Nakayama M, Iejima D, Akahori M, Kamei J, Goto A, Iwata T. Invest Ophthalmol Vis Sci. 2014 Sep 9;55(10):6514-23. doi: 10.1167/iovs.14-14453.
HTRA1 (high temperature requirement A serine peptidase 1) gene is transcriptionally regulated by insertion/deletion nucleotides located at the 3' end of the ARMS2 (age-related maculopathy susceptibility 2) gene in patients with age-related macular degeneration
Dry age-related macular degeneration (AMD) accounts for over 85% of AMD cases in the United States, whereas Japanese AMD patients predominantly progress to wet AMD or polypoidal choroidal vasculopathy. Recent genome-wide association studies have revealed a strong association between AMD and an insertion/deletion sequence between the ARMS2 (age-related maculopathy susceptibility 2) and HTRA1(high temperature requirement A serine peptidase 1) genes. Transcription regulator activity was localized in mouse retinas using heterozygous HtrA1 knock-out mice in which HtrA1 exon 1 was replaced with β-galactosidase cDNA, thereby resulting in dominant expression of the photoreceptors. The insertion/deletion sequence significantly induced HTRA1 transcription regulator activity in photoreceptor cell lines but not in retinal pigmented epithelium or other cell types. A deletion construct of the HTRA1 regulatory region indicated that potential transcriptional suppressors and activators surround the insertion/deletion sequence. Ten double-stranded DNA probes for this region were designed, three of which interacted with nuclear extracts from 661W cells in EMSA. Liquid chromatography-mass spectrometry (LC-MS/MS) of these EMSA bands subsequently identified a protein that bound the insertion/deletion sequence, LYRIC (lysine-rich CEACAM1 co-isolated) protein. In addition, induced pluripotent stem cells from wet AMD patients carrying the insertion/deletion sequence showed significant up-regulation of the HTRA1 transcript compared with controls. These data suggest that the insertion/deletion sequence alters the suppressor and activator cis-elements of HTRA1 and triggers sustained up-regulation of HTRA1. These results are consistent with a transgenic mouse model that ubiquitously overexpresses HtrA1 and exhibits characteristics similar to those of wet AMD patients.
Iejima D, Itabashi T, Kawamura Y, Noda T, Yuasa S, Fukuda K, Oka C, Iwata T. J Biol Chem. 2015 Jan 30;290(5):2784-97. doi: 10.1074/jbc.M114.593384. Epub 2014 Dec 17. PMID: 25519903
Genome Wide Association Study on Wet-Type AMD
AMD is considered a multifactorial disease with involvement of genetic, behavior, and environmental factors, and primarily affects the macular region of the retina. Clinical phenotypes of AMD are manifold. Small and hard drusen often appear in normal aged eyes, and do not necessarily cause AMD. In Caucasians, the early stage of AMD is associated with an increase in the number of large soft drusen and progresses to either the dry-form or wet-form of the disease. In contrast, Japanese patients predominantly exhibit wet-type AMD with choroidal neovascularization (CNV) and few or no drusen. Maruko et al. have classified wet-type AMD patients into three subgroups, namely typical wet-type AMD, PCV, and retinal angiomatous proliferation (RAP). From two hundred and eighty-nine Japanese patients examined with wet-type AMD, 35.3%, 54.7%, and 4.5% were diagnosed with typical AMD, PCV, and RAP, respectively. In the remaining 5.5% patients, one eye had PCV and the other eye had typical AMD. Thus PCV is the predominant subgroup of wet-type AMD in the Japanese population. PCV is characterized by branching of the choroidal vasculature basal to the RPE comprising various sized polypoidal structures connected to the branching vascular network, which can be clearly seen by indocyanine green angiography. PCV can be misdiagnosed as typical AMD if only fluorescein angiography is performed. The recent increase in prevalence of PCV is mainly due to the improvement of diagnostic methods. In most Japanese patients, typical wet-type AMD and PCV occur unilaterally (94.1% and 81.6% respectively) and show a male preponderance (71.6% and 77.8% respectively) consistent with studies of Eastern Asian patients in China and Korea. However, in Caucasians, PCV predominantly affects women and occurs bilaterally with a prevalence ranging from 4 to 14%, which is comparably lower than the Eastern Asian populations. On the other hand, it has been reported that the incidence of PCV in black individuals exceeds that of Asians. Although the phenotypic diversity of AMD has been speculated to be associated with differences in genetic background, this has not been clearly established.
Initial efforts to investigate the genetic basis of AMD utilized family studies. A concordance for AMD phenotypes in twins, and a higher risk of siblings of individuals with AMD have been reported. These early studies lead to genome-wide linkage analyses using microsatellite markers to search for chromosomal regions associated with affected individuals. Several candidate regions including 1q32 and 10q26 were confirmed by a meta-analysis. Progress in genotyping and sequencing technology extended detailed genetic association studies to the entire genome. Age-related eye disease studies (AREDS) of AMD case-control subjects using 100,000 SNPs resulted in the identification of four chromosomal regions significantly associated with the disease, namely CFH (1q32), ARMS2/HTRA1 (10q26), complement component 2 /complement factor B (C2/BF, 6p21), and complement component 3 (C3, 19p13), which is consistent with another genome wide association study recently reported by Swaroop, et al. It should be noted that these genome-wide scans have been conducted on subjects with both the dry and wet form of AMD, with the majority of cases representing the dry form of the disease. However, Zang et al. have identified 34 SNPs which were associated with AMD at p value of less than 10-6 in the AREDS Caucasian cohort having typical wet-type AMD. They showed that 1q32 and 10q26 were also significantly associated with typical wet-type AMD. To date, there are no genome wide genetic studies reported for PCV.
Direct examinations of SNPs in chromosomal regions identified by genome-wide linkage analysis showed that two genomic loci 1q32 and 10q26 including the CFH, ARMS2, and HTRA1 genes were associated with AMD in Caucasians and individuals of Hong Kong. The association between AMD and three SNPs in these gene regions, namely rs1061170 (CFH), rs10490924 (ARMS2), and rs11200638 (HTRA1), were verified by a number of research groups around the world. We also confirmed the association of rs10490924 and rs11200638 with typical wet-type AMD in the Japanese cohort. From our results and others, rs10490924 and rs11200638 have been shown to strongly associate with dry-type AMD, typical wet-type AMD, and PCV. It is still unclear how these SNPs contribute to the development of different types of AMD. On the other hand, no association was confirmed for rs1061170 in Japanese AMD. This is probably due to the lower allele frequency of 0.07 ± 0.02 for this variant in Eastern Asian population compared to the higher frequency of 0.34 ± 0.03 in Caucasians. However, rs800292, another coding SNP in the CFH gene region originally associated with AMD in Caucasians, has been shown to associate with typical wet-type AMD and PCV in Japanese and Chinese populations. Thus, there is a clear difference in genetic risk for AMD based on ethnicity.
Since the association between the CFH gene and AMD has been established, other components of the complement pathway have been thoroughly examined in Caucasian populations. Among them, the 19p13, 6p21 and 4q25 loci, including the C2, BF, C3, and complement factor I (CFI) genes, show strong association with AMD. However, it still unclear whether CFI or the nearby PLA2G12A gene is associated with the disease. Lee et al. analyzed the same AMD associated SNPs in the C2/BF gene region and reported that there were no differences between
Chinese PCV patients and control groups. Similar results were obtained for PCV in Japanese population for SNPs in the C2/BF gene region. However, in this study, significant association of disease-protective haplotype was observed. There is currently no evidence that the C2/BF gene region is a risk for wet-type AMD or the C3 gene region a risk for typical wet-type AMD and PCV for Eastern Asians. With the exception of 1q32, 10q26, 19p13, 6p21, and 4q25 regions, association of other loci with AMD pathogenesis reported by some genome wide genetic studies remains unclear. These SNP variants may be ethnic specific as is the case for CFH. To investigate the involvement of genetic factors in Japanese patients who progressed to typical wet-type AMD and PCV, over 500,568 SNPs covering ARMS2/HTRA1, CFH, C2/BF, C3, and CFI and other regions were genotyped using Affymetrix Human Mapping 500K Arrays and TaqMan assay.
Goto A, Akahori A, Okamoto H, Minami M, Terauchi N, Haruhata Y, Obazawa M, Noda T, Honda M, Mizota A, Tanaka M, Hayashi T, Tanito M, Ogata N, and Iwata T. Genetic analysis of typical wet-type age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese population. Journal of Ocular Biology, Disease, and Informatics 2:164-175 (2009)
Yoshida T, Wan AD, Zhang H, Sakamoto R, Okamoto H, Minami M, Obazawa M, Mizota A, Tanaka M, Saito Y, Takagi I, Hoh J, and Iwata T. HTRA1 Promoter Polymorphism Predisposes Japanese to AMD. Molecular Vision 13:545-548 (2007)
Okamoto H, Umeda S, Obazawa M, Minami M, Noda T, Mizota A, Honda M, Tanaka M, Koyama R, Takagi I, Sakamoto Y, Saito Y, Miyake Y, and Iwata T. Complement Factor H Polymorphisms in Japanese Population with Age-Related Macular Degeneration. Molecular Vision 12:156-158 (2006)
Characterization of Drusen Cynomolgus Monkey
 Primate is a member of the biological order that contains prosimians (lemurs, lorises, galagos, and tarsiers) and simians (monkeys and apes). Simians are divided into two groups, the platyrrhines (new world monkeys) of South and Central America and the catarrhine monkeys of Africa and Southeastern Asia. The new world monkeys include the capuchin, howler, and squirrel monkeys, and the catarrhines include the old world monkeys (baboons and macaques) and the apes. The non-human primates preferred for vision research are the macaque monkeys
Primate is a member of the biological order that contains prosimians (lemurs, lorises, galagos, and tarsiers) and simians (monkeys and apes). Simians are divided into two groups, the platyrrhines (new world monkeys) of South and Central America and the catarrhine monkeys of Africa and Southeastern Asia. The new world monkeys include the capuchin, howler, and squirrel monkeys, and the catarrhines include the old world monkeys (baboons and macaques) and the apes. The non-human primates preferred for vision research are the macaque monkeys
(Macaca mulatta, Macaca fascicularis, and Macaca fuscata). Macaques rely on three-color (red, green, and blue) stereoscopic vision, the dominant sensory system, with larger brains compared to other mammals for accurate information processing. Macaques have slower rates of development than other similarly sized mammals, and reach maturity later but have longer lifespans. These characteristics mimic the human development of age-related eye diseases.
When working with macula related diseases, animal model with well-defined fovea is preferred. A search for monkey line affected with macular degeneration has been persistent for decades. A monkey with macular degeneration was first described by Stafford et al. in 1974. More than 6% of the elderly monkeys they examined showed pigmentary disorders and drusen-like spots. El-Mofty et al. also reported that the incidence of maculopathy was high as 50% in a colony of rhesus monkeys at the Caribbean Primate Research Center of the University of Puerto Rico.
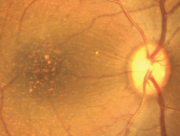
 Dawson et al. examined 272 eyes in 136 rhesus monkeys during 1986-1988 in the closed Cayo Santiago colony of the Caribbean Primate Research Center of the University of Puerto Rico. The fundi were examined and photographed for all eyes and fluorescein angiography was also performed in some eyes. Selected cases were evaluated for 'acuity' loss by recording of pattern-evoked retinal and cortical signals. Light and electron microscopy were used to evaluate the pigment epithelium of some animals. Among examined eye 38% of all eyes had posterior pole drusen. Incidence was highly age-related. When late-stage lesions were found, no CNV was observed, but late hyperfluorescence was consistent with degenerative scarring and atrophy. Electrophysiology demonstrated moderately reduced acuity in the presence of numerous macular drusen. The electrooculograms were below normal and the histopathology showed changes identical to those reported in human AMD.
Dawson et al. examined 272 eyes in 136 rhesus monkeys during 1986-1988 in the closed Cayo Santiago colony of the Caribbean Primate Research Center of the University of Puerto Rico. The fundi were examined and photographed for all eyes and fluorescein angiography was also performed in some eyes. Selected cases were evaluated for 'acuity' loss by recording of pattern-evoked retinal and cortical signals. Light and electron microscopy were used to evaluate the pigment epithelium of some animals. Among examined eye 38% of all eyes had posterior pole drusen. Incidence was highly age-related. When late-stage lesions were found, no CNV was observed, but late hyperfluorescence was consistent with degenerative scarring and atrophy. Electrophysiology demonstrated moderately reduced acuity in the presence of numerous macular drusen. The electrooculograms were below normal and the histopathology showed changes identical to those reported in human AMD.
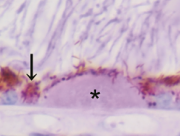
At the Tsukuba Primate Research Center (TPRC) in Japan, a single cynomolgus monkey (Macaca fascicularis) with a large number of small drusen around the macular region was found. This single affected monkey has mated to a large pedigree of more than 100 affect and 300 unaffected monkeys. Drusen were observed in the macular region as early as one year after birth, and the numbers increased and spread toward the peripheral retina throughout life. No histological abnormalities have been found in the retina, retinal vessels, or choroidal vasculatures of the eyes with drusen. Another sporadic aged cynomolgus monkey with drusen was found in Simian Conservation Breeding and Research Center (SICONBREC) in the Philippines. More than 10% of the elderly monkeys had soft drusen. Immunohistochemical and proteomic analyses of the drusen from these monkeys showed similar to human. These drusen contained protein molecules that mediate inflammatory and immune processes, which included immunoglobulins, components of complement pathway, modulators for complement activation (e.g., vitronectin, clusterin, membrane cofactor protein, and complement receptor-1), molecules involved in the acute-phase response to inflammation (e.g., amyloid P component, α1-antitrypsin, and apolipoprotein E), major histocompatibility complex class II antigens, and HLA-DR antigens. Cellular components have also been identified in drusen, including RPE debris, lipofuscin, and melanin, as well as processes of choroidal dendritic cells, which are felt to contribute to the inflammatory response. In addition to immune components, a number of other proteins were found in drusen. These appear to be vitronectin, clusterin, TIMP-3, serum amyloid P component, apolipoprotein E, IgG, Factor X, crystallins, EEFMP1, and amyloid-beta. The presence of immunoreactive proteins and oxidative modified proteins implicated that both oxidation and immune functions in the pathogenesis of AMD.
 The affected monkeys from TPRC were further used to test new drug, which suppress complement activation in the retina, to delay/cure AMD. To test the effect of long term suppression of complement activation in the retina, an cyclic analogue (Ac-I[CV(1MeW)QDWGAHRC]T-NH2) of the small cyclic synthetic peptide compstatin was intravitreally injected into 8 affected monkey intravitreally injected at different dose and intervals. Four affected monkeys were injected at 50 μg dose at one week interval. Fifty microgram dose were dissolved in 100 μl of saline solution, filtrated and intravitreally injected using 30G needles. After 6 month of injection, diffusion of drusen in the macula and by 9 month partial disappearance of drusen was observed in all 4 monkeys. This preliminary experiment has shown reversal of drusen formation by suppression of complement activation. The information should benefit for development of improved drug and therapy for future AMD prevention.
The affected monkeys from TPRC were further used to test new drug, which suppress complement activation in the retina, to delay/cure AMD. To test the effect of long term suppression of complement activation in the retina, an cyclic analogue (Ac-I[CV(1MeW)QDWGAHRC]T-NH2) of the small cyclic synthetic peptide compstatin was intravitreally injected into 8 affected monkey intravitreally injected at different dose and intervals. Four affected monkeys were injected at 50 μg dose at one week interval. Fifty microgram dose were dissolved in 100 μl of saline solution, filtrated and intravitreally injected using 30G needles. After 6 month of injection, diffusion of drusen in the macula and by 9 month partial disappearance of drusen was observed in all 4 monkeys. This preliminary experiment has shown reversal of drusen formation by suppression of complement activation. The information should benefit for development of improved drug and therapy for future AMD prevention.
The macaque genome project, which was completed in 2007 generated large amount genome information including the microsatellite markers for linkage study. Currently 240 loci of the cynomolgus monkey are being investigated to try to identify the disease causing gene and to understand the biological pathways leading to complement activation.
The eyes of monkey are structurally similar to human eyes which make them extremely valuable for macular degeneration studies. However, there are limitations in using this species over other laboratory animals. Monkeys have a relatively longer life span, have a longer gestation period, have a lower birth numbers resulting in a slower rate of expanding the pedigree, more difficult to genetically manipulate, and the cost of maintenance is significantly higher. In the other laboratory animals, the differences in the eye structure have been considered to be a disadvantage for using them as AMD models. However, they are easier to maintain and less expensive. This has made the development of a mouse model of AMD very attractive, and a number of mouse AMD models have been reported recently.
Chi Z-L, Yoshida T, Lambris JD, and Iwata T. Suppression of drusen formation by compstatin, a peptide inhibitor of complement C3 activation, on Cynomolgus monkey with early-onset macular degeneration. Currrent Topics on Complement and Eye Disease, Advances in Experimental Medicine and Biology 703:127-135 (2010)
Umeda S, Suzuki MT , Okamoto H, Ono F, Mizota A, Terao K, Yoshikawa Y, Tanaka Y, and Iwata T. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (Macaca fascicularis). FASEB Journal 19:1683-1685 (2005)
Umeda S, Ayyagari R, Allikmets R, Suzuki MT, Karoukis AJ, Ambasudhan R, Zernant J, Okamoto H, Ono F, Terao K, Atsushi M, Yoshikawa Y, Tanaka Y, and Iwata T. Early onset macular degeneration with drusen in a cynomolgus monkey (Macaca fascicularis) pedigree caused by a novel gene mutation. Investive Ophthalmology and Visual Science 46:683-691 (2005)
Umeda S, Suzuki MT, Yoshikawa Y, Iwata F, Fujiki K, Kanai A, Sanuki N, Tanaka Y, and Iwata T. Cloning and Characterization of ELVLO4 Gene in Cynomolgus (Macaca fascicularis) Monkey. Experimental Animal 52:(2) (2003)

