Animal Models for Retinal Diseases
CCT2 Mutations Evoke Leber Congenital Amaurosis due to Chaperone Complex Instability
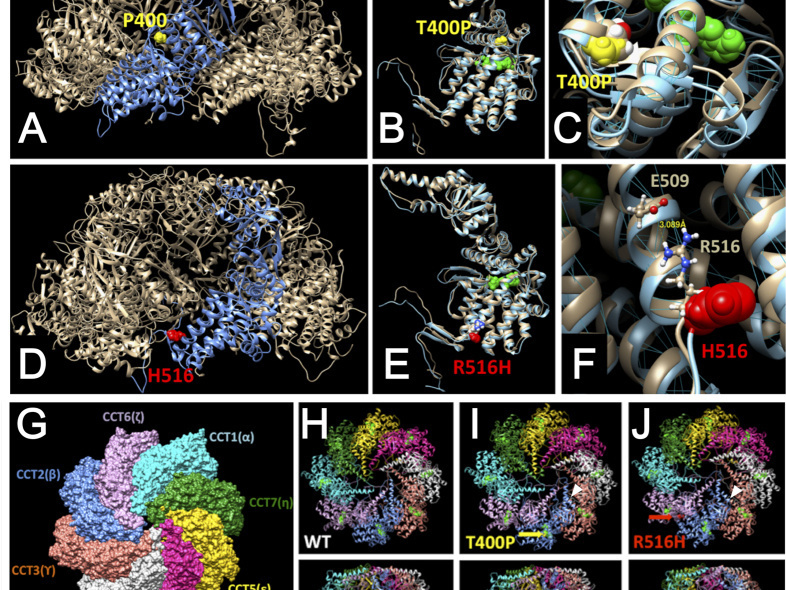 Cytoplasmic localization of Flag-tagged C21orf2 wild type and mutant proteins. Insert shows overlapping Flag (green) and Tubulin (red) immunostaining.Leber congenital amaurosis (LCA) is a hereditary early-onset retinal dystrophy that is accompanied by severe macular degeneration. In this study, novel compound heterozygous mutations were identified as LCA-causative in chaperonin-containing TCP-1, subunit 2 (CCT2), a gene that encodes the molecular chaperone protein, CCTβ. The zebrafish mutants of CCTβ are known to exhibit the eye phenotype while its mutation and association with human disease have been unknown. The CCT proteins (CCT α-θ) forms ring complex for its chaperon function. The LCA mutants of CCTβ, T400P and R516H, are biochemically instable and the affinity for the adjacent subunit, CCTγ, was affected distinctly in both mutants. The patient-derived induced pluripotent stem cells (iPSCs), carrying these CCTβ mutants, were less proliferative than the control iPSCs. Decreased proliferation under Cct2 knockdown in 661W cells was significantly rescued by wild-type CCTβ expression. However, the expression of T400P and R516H didn't exhibit the significant effect. In mouse retina, both CCTβ and CCTγ are expressed in the retinal ganglion cells and connecting cilium of photoreceptor cells. The Cct2 knockdown decreased its major client protein, transducing β1 (Gβ1). Here we report the novel LCA mutations in CCTβ and the impact of chaperon disability by these mutations in cellular biology.
Cytoplasmic localization of Flag-tagged C21orf2 wild type and mutant proteins. Insert shows overlapping Flag (green) and Tubulin (red) immunostaining.Leber congenital amaurosis (LCA) is a hereditary early-onset retinal dystrophy that is accompanied by severe macular degeneration. In this study, novel compound heterozygous mutations were identified as LCA-causative in chaperonin-containing TCP-1, subunit 2 (CCT2), a gene that encodes the molecular chaperone protein, CCTβ. The zebrafish mutants of CCTβ are known to exhibit the eye phenotype while its mutation and association with human disease have been unknown. The CCT proteins (CCT α-θ) forms ring complex for its chaperon function. The LCA mutants of CCTβ, T400P and R516H, are biochemically instable and the affinity for the adjacent subunit, CCTγ, was affected distinctly in both mutants. The patient-derived induced pluripotent stem cells (iPSCs), carrying these CCTβ mutants, were less proliferative than the control iPSCs. Decreased proliferation under Cct2 knockdown in 661W cells was significantly rescued by wild-type CCTβ expression. However, the expression of T400P and R516H didn't exhibit the significant effect. In mouse retina, both CCTβ and CCTγ are expressed in the retinal ganglion cells and connecting cilium of photoreceptor cells. The Cct2 knockdown decreased its major client protein, transducing β1 (Gβ1). Here we report the novel LCA mutations in CCTβ and the impact of chaperon disability by these mutations in cellular biology.
Minegishi Y, Sheng X, Yoshitake K, Sergeev Y, Iejima D, Shibagaki Y, Monma N, Ikeo K, Furuno M, Zhuang W, Liu Y, Rong W, Hattori S, Iwata T.
Sci Rep. 2016 Sep 20;6:33742. doi: 10.1038/srep33742. PMID: 27645772
METTL23 mutation alters histone H3R17 methylation in normal-tension glaucoma
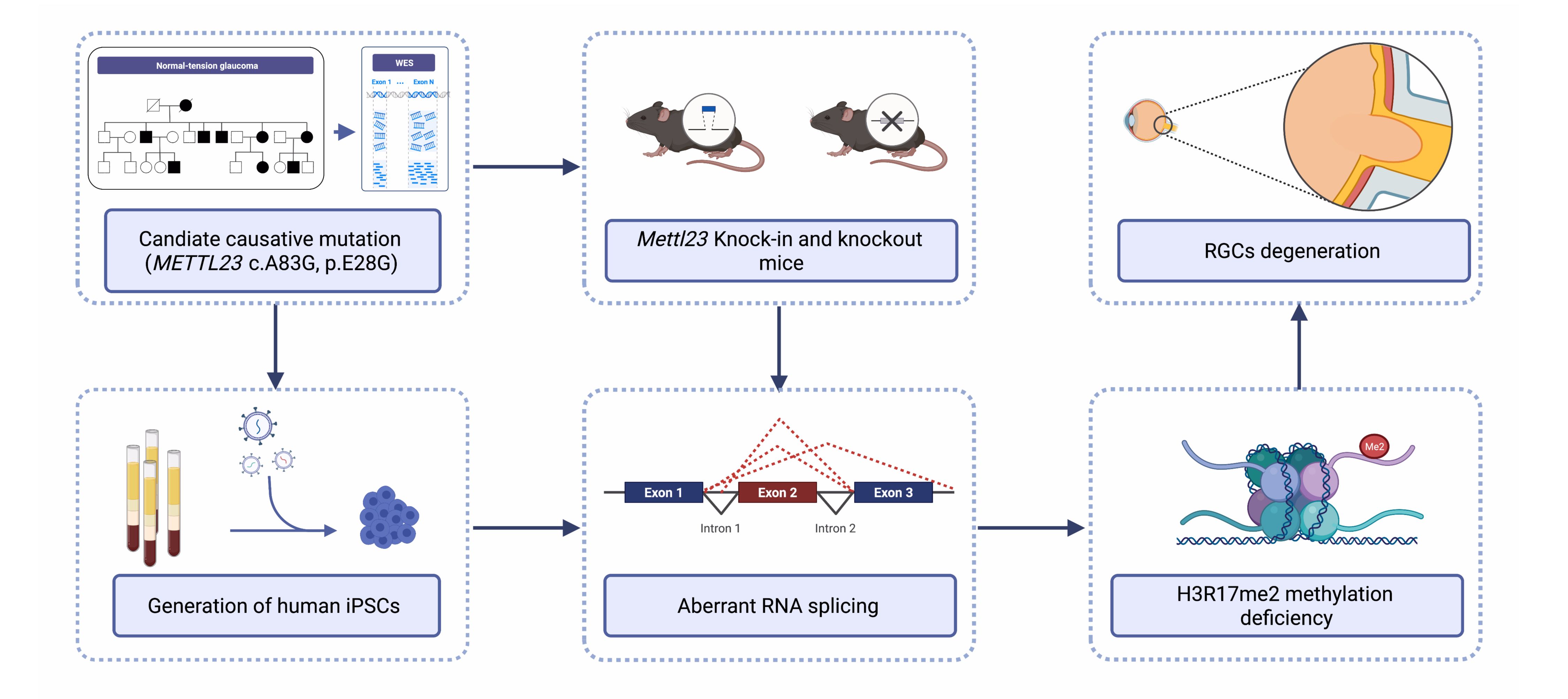 Normal-tension glaucoma (NTG) is a heterogeneous disease characterized by retinal ganglion cell (RGC) death leading to cupping of the optic nerve head and visual field loss at normal intraocular pressure (IOP). The pathogenesis of NTG remains unclear. Here, we described a single nucleotide mutation in exon 2 of the methyltransferase like 23 (METTL23) gene identified in a three-generation Japanese NTG family. This mutation caused METTL23 mRNA aberrant splicing, which abolished normal protein production and altered subcellular localization. Mettl23 knock-in (Mettl23+/G & Mettl23G/G) and knockout (Mettl23+/- & Mettl23-/-) mice developed a glaucoma phenotype without elevated IOP. METTL23 is a histone arginine methyltransferase expressed in murine and macaque RGCs. However, the novel mutation reduced Mettl23 expression in RGCs of Mettl23G/G mice, which recapitulated both clinical and biological phenotypes. Moreover, our findings demonstrated that Mettl23 catalyzed the dimethylation of H3R17 in the retina, and was required for the transcription of pS2, an estrogen receptor α target gene that was critical to RGC homeostasis through the negative regulation of NF-κB-mediated TNF-α/IL-1β feedback. These findings suggest an etiologic role of METTL23 in NTG with tissue-specific pathology.
Normal-tension glaucoma (NTG) is a heterogeneous disease characterized by retinal ganglion cell (RGC) death leading to cupping of the optic nerve head and visual field loss at normal intraocular pressure (IOP). The pathogenesis of NTG remains unclear. Here, we described a single nucleotide mutation in exon 2 of the methyltransferase like 23 (METTL23) gene identified in a three-generation Japanese NTG family. This mutation caused METTL23 mRNA aberrant splicing, which abolished normal protein production and altered subcellular localization. Mettl23 knock-in (Mettl23+/G & Mettl23G/G) and knockout (Mettl23+/- & Mettl23-/-) mice developed a glaucoma phenotype without elevated IOP. METTL23 is a histone arginine methyltransferase expressed in murine and macaque RGCs. However, the novel mutation reduced Mettl23 expression in RGCs of Mettl23G/G mice, which recapitulated both clinical and biological phenotypes. Moreover, our findings demonstrated that Mettl23 catalyzed the dimethylation of H3R17 in the retina, and was required for the transcription of pS2, an estrogen receptor α target gene that was critical to RGC homeostasis through the negative regulation of NF-κB-mediated TNF-α/IL-1β feedback. These findings suggest an etiologic role of METTL23 in NTG with tissue-specific pathology.
Pan Y, Suga A, Kimura I, Kimura C, Minegishi Y, Nakayama M, Yoshitake K, Iejima D, Minematsu N, Yamamoto M, Mabuchi F, Takamoto M, Shiga Y, Araie M, Kashiwagi K, Aihara M, Nakazawa T, Iwata T.
J Clin Invest. 2022 Sep 13:e153589. doi: 10.1172/JCI153589. Online ahead of print.PMID: 36099048
Novel mutations in malonyl-CoA-acyl carrier protein transacylase provoke autosomal recessive optic neuropathy
 Inherited optic neuropathies are rare eye diseases of optic nerve dysfunction that present in various genetic forms. Previously, mutation in three genes encoding mitochondrial proteins has been implicated in autosomal recessive forms of optic atrophy that involve progressive degeneration of optic nerve and retinal ganglion cells (RGC). Using whole exome analysis, a novel double homozygous mutation p.L81R and pR212W in malonyl CoA-acyl carrier protein transacylase (MCAT), a mitochondrial protein involved in fatty acid biosynthesis, has now been identified as responsible for an autosomal recessive optic neuropathy from a Chinese consanguineous family. MCAT is expressed in RGC that are rich in mitochondria. The disease variants lead to structurally unstable MCAT protein with significantly reduced intracellular expression. RGC-specific knockdown of Mcat in mice, lead to an attenuated retinal neurofiber layer, that resembles the phenotype of optic neuropathy. These results indicated that MCAT plays an essential role in mitochondrial function and maintenance of RGC axons, while novel MCAT p.L81R and p.R212W mutations can lead to optic neuropathy.
Inherited optic neuropathies are rare eye diseases of optic nerve dysfunction that present in various genetic forms. Previously, mutation in three genes encoding mitochondrial proteins has been implicated in autosomal recessive forms of optic atrophy that involve progressive degeneration of optic nerve and retinal ganglion cells (RGC). Using whole exome analysis, a novel double homozygous mutation p.L81R and pR212W in malonyl CoA-acyl carrier protein transacylase (MCAT), a mitochondrial protein involved in fatty acid biosynthesis, has now been identified as responsible for an autosomal recessive optic neuropathy from a Chinese consanguineous family. MCAT is expressed in RGC that are rich in mitochondria. The disease variants lead to structurally unstable MCAT protein with significantly reduced intracellular expression. RGC-specific knockdown of Mcat in mice, lead to an attenuated retinal neurofiber layer, that resembles the phenotype of optic neuropathy. These results indicated that MCAT plays an essential role in mitochondrial function and maintenance of RGC axons, while novel MCAT p.L81R and p.R212W mutations can lead to optic neuropathy.
Li H, Yuan S, Minegishi Y, Suga A, Yoshitake K, Sheng X, Ye J, Smith S, Bunkoczi G, Yamamoto M, Iwata T. Novel mutations in malonyl-CoA-acyl carrier protein transacylase provoke autosomal recessive optic neuropathy. Hum Mol Genet. 2020 Jan 9. pii: ddz311. doi: 10.1093/hmg/ddz311. [Epub ahead of print] PubMed PMID: 31915829.
Significance of optineurin mutations in glaucoma and other diseases
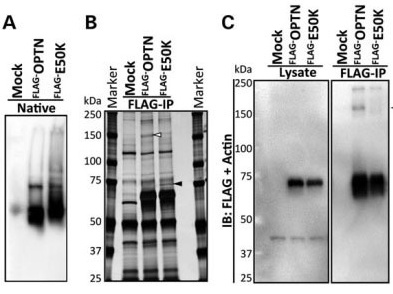 Glaucoma is one of the leading causes of bilateral blindness, affecting nearly 57 million people worldwide. Glaucoma is characterized by a progressive loss of retinal ganglion cells and is often associated with intraocular pressure (IOP). Normal tension glaucoma (NTG), marked by normal IOP but progressive glaucoma, is incompletely understood. In 2002, Sarfarazi et al. identified FIP-2 gene mutations responsible for hereditary NTG, renaming this gene "optineurin" (OPTN). Further investigations by multiple groups worldwide showed that OPTN is involved in several critical cellular functions, such as NF-κB regulation, autophagy, and vesicle transport. Recently, OPTN mutations were found to cause amyotrophic lateral sclerosis (ALS). Surprisingly, a mutation in the OPTN interacting protein, i.e., the duplication of TANK binding protein 1 (TBK1) gene, also can cause both NTG and ALS. These phenotypically distinct neuronal diseases are now merging into one common pathological mechanism by these two genes. TBK1 inhibition has emerged as a potential therapy for NTG. In this manuscript, we focus on the OPTN E50K mutation, the most common mutation for NTG, to describe the molecular mechanism of NTG by expressing a mutant Optn gene in cells and genetically modified mice. Patient iPS cells were developed and differentiated into neural cells to observe abnormal behavior and the impact of the E50K mutation. These in vitro studies were further extended to identify the inhibitors BX795 and amlexanox, which have the potential to reverse the disease-causing phenomenon in patient's neural cells. Here we show for the first time that amlexanox protects RGCs in Optn E50K knock-in mice.
Glaucoma is one of the leading causes of bilateral blindness, affecting nearly 57 million people worldwide. Glaucoma is characterized by a progressive loss of retinal ganglion cells and is often associated with intraocular pressure (IOP). Normal tension glaucoma (NTG), marked by normal IOP but progressive glaucoma, is incompletely understood. In 2002, Sarfarazi et al. identified FIP-2 gene mutations responsible for hereditary NTG, renaming this gene "optineurin" (OPTN). Further investigations by multiple groups worldwide showed that OPTN is involved in several critical cellular functions, such as NF-κB regulation, autophagy, and vesicle transport. Recently, OPTN mutations were found to cause amyotrophic lateral sclerosis (ALS). Surprisingly, a mutation in the OPTN interacting protein, i.e., the duplication of TANK binding protein 1 (TBK1) gene, also can cause both NTG and ALS. These phenotypically distinct neuronal diseases are now merging into one common pathological mechanism by these two genes. TBK1 inhibition has emerged as a potential therapy for NTG. In this manuscript, we focus on the OPTN E50K mutation, the most common mutation for NTG, to describe the molecular mechanism of NTG by expressing a mutant Optn gene in cells and genetically modified mice. Patient iPS cells were developed and differentiated into neural cells to observe abnormal behavior and the impact of the E50K mutation. These in vitro studies were further extended to identify the inhibitors BX795 and amlexanox, which have the potential to reverse the disease-causing phenomenon in patient's neural cells. Here we show for the first time that amlexanox protects RGCs in Optn E50K knock-in mice.
Minegishi Y, Nakayama M, Iejima D, Kawase K, Iwata T.
Prog Retin Eye Res. 2016 Nov;55:149-181. doi: 0.1016/j.preteyeres.2016.08.002. Epub 2016 Sep 29. PMID: 27693724
Overexpression of HtrA1 and exposure to mainstream cigarette smoke leads to choroidal neovascularization and subretinal deposits in aged mice
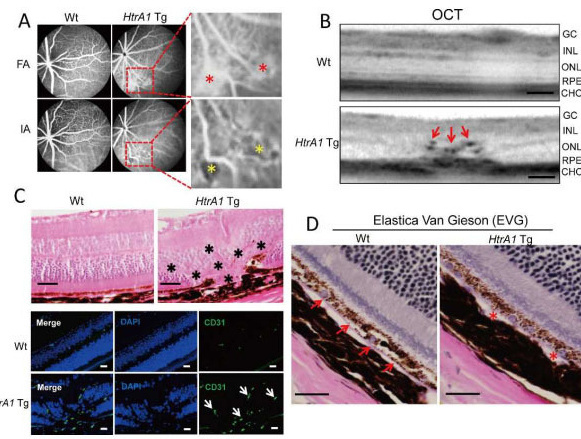 We determined the function of ARMS2 and HtrA1 in the choroid and retina using transgenic (Tg) mice and evaluated the effects of mainstream cigarette smoke on these mice. The chicken actin promoter (CAG) was used to drive mouse HtrA1, human ARMS2, and ARMS2 (A69S) expression in the entire body of a mouse for one year. Fundus observations were performed with a Spectralis HRA+ optical coherence tomograph (OCT). Eyes were sectioned, stained with hematoxylin and eosin (H&E), and analyzed with immunohistochemistry. Mice were exposed to cigarette smoke for 30 min/d, 5 d/wk for 12 weeks using a mainstream smoking chamber (INH06-CIGR02A, MIPS). After 12 weeks, fundus observations and pathological analyses were performed. Approximately 18.2% of 12-month-old HtrA1 Tg mice exhibited choroidal neovascularization (CNV) by OCT and positive immunostaining with anti-CD31 and anti-fibronectin antibodies. Furthermore, elastic van Gieson (EVG) staining showed Bruch's membrane damage in HtrA1 Tg mice. No retinal changes were observed in ARMS2 and ARMS2 (A69S) Tg mice. A total of 12 weeks of exposure to mainstream cigarette smoke led to CNV rates of 7.7% for wild type (Wt) mice and 20% for HtrA1 Tg mice, but had no effect on ARMS2 Tg mice. In addition, abnormal deposits were observed between photoreceptor cells and the RPE in an HtrA1 Tg mouse exposed to mainstream cigarette smoke. The HtrA1 overexpression and mainstream cigarette smoke can independently lead to CNV. The HtrA1 gene is a strong risk factor for wet AMD, but not all of the HtrA1 Tg mice developed CNV, suggesting that CNV development depends on multiple risk factors.
We determined the function of ARMS2 and HtrA1 in the choroid and retina using transgenic (Tg) mice and evaluated the effects of mainstream cigarette smoke on these mice. The chicken actin promoter (CAG) was used to drive mouse HtrA1, human ARMS2, and ARMS2 (A69S) expression in the entire body of a mouse for one year. Fundus observations were performed with a Spectralis HRA+ optical coherence tomograph (OCT). Eyes were sectioned, stained with hematoxylin and eosin (H&E), and analyzed with immunohistochemistry. Mice were exposed to cigarette smoke for 30 min/d, 5 d/wk for 12 weeks using a mainstream smoking chamber (INH06-CIGR02A, MIPS). After 12 weeks, fundus observations and pathological analyses were performed. Approximately 18.2% of 12-month-old HtrA1 Tg mice exhibited choroidal neovascularization (CNV) by OCT and positive immunostaining with anti-CD31 and anti-fibronectin antibodies. Furthermore, elastic van Gieson (EVG) staining showed Bruch's membrane damage in HtrA1 Tg mice. No retinal changes were observed in ARMS2 and ARMS2 (A69S) Tg mice. A total of 12 weeks of exposure to mainstream cigarette smoke led to CNV rates of 7.7% for wild type (Wt) mice and 20% for HtrA1 Tg mice, but had no effect on ARMS2 Tg mice. In addition, abnormal deposits were observed between photoreceptor cells and the RPE in an HtrA1 Tg mouse exposed to mainstream cigarette smoke. The HtrA1 overexpression and mainstream cigarette smoke can independently lead to CNV. The HtrA1 gene is a strong risk factor for wet AMD, but not all of the HtrA1 Tg mice developed CNV, suggesting that CNV development depends on multiple risk factors.
Nakayama M, Iejima D, Akahori M, Kamei J, Goto A, Iwata T.
Invest Ophthalmol Vis Sci. 2014 Sep 9;55(10):6514-23. doi: 10.1167/iovs.14-14453.
Characterization of Drusen Cynomolgus Monkey
 Primate is a member of the biological order that contains prosimians (lemurs, lorises, galagos, and tarsiers) and simians (monkeys and apes). Simians are divided into two groups, the platyrrhines (new world monkeys) of South and Central America and the catarrhine monkeys of Africa and Southeastern Asia. The new world monkeys include the capuchin, howler, and squirrel monkeys, and the catarrhines include the old world monkeys (baboons and macaques) and the apes. The non-human primates preferred for vision research are the macaque monkeys
Primate is a member of the biological order that contains prosimians (lemurs, lorises, galagos, and tarsiers) and simians (monkeys and apes). Simians are divided into two groups, the platyrrhines (new world monkeys) of South and Central America and the catarrhine monkeys of Africa and Southeastern Asia. The new world monkeys include the capuchin, howler, and squirrel monkeys, and the catarrhines include the old world monkeys (baboons and macaques) and the apes. The non-human primates preferred for vision research are the macaque monkeys
(Macaca mulatta, Macaca fascicularis, and Macaca fuscata). Macaques rely on three-color (red, green, and blue) stereoscopic vision, the dominant sensory system, with larger brains compared to other mammals for accurate information processing. Macaques have slower rates of development than other similarly sized mammals, and reach maturity later but have longer lifespans. These characteristics mimic the human development of age-related eye diseases.
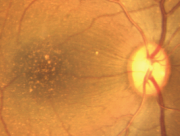
When working with macula related diseases, animal model with well-defined fovea is preferred. A search for monkey line affected with macular degeneration has been persistent for decades. A monkey with macular degeneration was first described by Stafford et al. in 1974. More than 6% of the elderly monkeys they examined showed pigmentary disorders and drusen-like spots. El-Mofty et al. also reported that the incidence of maculopathy was high as 50% in a colony of rhesus monkeys at the Caribbean Primate Research Center of the University of Puerto Rico.
 Dawson et al. examined 272 eyes in 136 rhesus monkeys during 1986-1988 in the closed Cayo Santiago colony of the Caribbean Primate Research Center of the University of Puerto Rico. The fundi were examined and photographed for all eyes and fluorescein angiography was also performed in some eyes. Selected cases were evaluated for 'acuity' loss by recording of pattern-evoked retinal and cortical signals. Light and electron microscopy were used to evaluate the pigment epithelium of some animals. Among examined eye 38% of all eyes had posterior pole drusen. Incidence was highly age-related. When late-stage lesions were found, no CNV was observed, but late hyperfluorescence was consistent with degenerative scarring and atrophy. Electrophysiology demonstrated moderately reduced acuity in the presence of numerous macular drusen. The electrooculograms were below normal and the histopathology showed changes identical to those reported in human AMD.
Dawson et al. examined 272 eyes in 136 rhesus monkeys during 1986-1988 in the closed Cayo Santiago colony of the Caribbean Primate Research Center of the University of Puerto Rico. The fundi were examined and photographed for all eyes and fluorescein angiography was also performed in some eyes. Selected cases were evaluated for 'acuity' loss by recording of pattern-evoked retinal and cortical signals. Light and electron microscopy were used to evaluate the pigment epithelium of some animals. Among examined eye 38% of all eyes had posterior pole drusen. Incidence was highly age-related. When late-stage lesions were found, no CNV was observed, but late hyperfluorescence was consistent with degenerative scarring and atrophy. Electrophysiology demonstrated moderately reduced acuity in the presence of numerous macular drusen. The electrooculograms were below normal and the histopathology showed changes identical to those reported in human AMD.
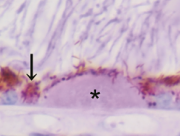
At the Tsukuba Primate Research Center (TPRC) in Japan, a single cynomolgus monkey (Macaca fascicularis) with a large number of small drusen around the macular region was found. This single affected monkey has mated to a large pedigree of more than 100 affect and 300 unaffected monkeys. Drusen were observed in the macular region as early as one year after birth, and the numbers increased and spread toward the peripheral retina throughout life. No histological abnormalities have been found in the retina, retinal vessels, or choroidal vasculatures of the eyes with drusen. Another sporadic aged cynomolgus monkey with drusen was found in Simian Conservation Breeding and Research Center (SICONBREC) in the Philippines. More than 10% of the elderly monkeys had soft drusen. Immunohistochemical and proteomic analyses of the drusen from these monkeys showed similar to human. These drusen contained protein molecules that mediate inflammatory and immune processes, which included immunoglobulins, components of complement pathway, modulators for complement activation (e.g., vitronectin, clusterin, membrane cofactor protein, and complement receptor-1), molecules involved in the acute-phase response to inflammation (e.g., amyloid P component, α1-antitrypsin, and apolipoprotein E), major histocompatibility complex class II antigens, and HLA-DR antigens. Cellular components have also been identified in drusen, including RPE debris, lipofuscin, and melanin, as well as processes of choroidal dendritic cells, which are felt to contribute to the inflammatory response. In addition to immune components, a number of other proteins were found in drusen. These appear to be vitronectin, clusterin, TIMP-3, serum amyloid P component, apolipoprotein E, IgG, Factor X, crystallins, EEFMP1, and amyloid-beta. The presence of immunoreactive proteins and oxidative modified proteins implicated that both oxidation and immune functions in the pathogenesis of AMD.
 The affected monkeys from TPRC were further used to test new drug, which suppress complement activation in the retina, to delay/cure AMD. To test the effect of long term suppression of complement activation in the retina, an cyclic analogue (Ac-I[CV(1MeW)QDWGAHRC]T-NH2) of the small cyclic synthetic peptide compstatin was intravitreally injected into 8 affected monkey intravitreally injected at different dose and intervals. Four affected monkeys were injected at 50 μg dose at one week interval. Fifty microgram dose were dissolved in 100 μl of saline solution, filtrated and intravitreally injected using 30G needles. After 6 month of injection, diffusion of drusen in the macula and by 9 month partial disappearance of drusen was observed in all 4 monkeys. This preliminary experiment has shown reversal of drusen formation by suppression of complement activation. The information should benefit for development of improved drug and therapy for future AMD prevention.
The affected monkeys from TPRC were further used to test new drug, which suppress complement activation in the retina, to delay/cure AMD. To test the effect of long term suppression of complement activation in the retina, an cyclic analogue (Ac-I[CV(1MeW)QDWGAHRC]T-NH2) of the small cyclic synthetic peptide compstatin was intravitreally injected into 8 affected monkey intravitreally injected at different dose and intervals. Four affected monkeys were injected at 50 μg dose at one week interval. Fifty microgram dose were dissolved in 100 μl of saline solution, filtrated and intravitreally injected using 30G needles. After 6 month of injection, diffusion of drusen in the macula and by 9 month partial disappearance of drusen was observed in all 4 monkeys. This preliminary experiment has shown reversal of drusen formation by suppression of complement activation. The information should benefit for development of improved drug and therapy for future AMD prevention.
The macaque genome project, which was completed in 2007 generated large amount genome information including the microsatellite markers for linkage study. Currently 240 loci of the cynomolgus monkey are being investigated to try to identify the disease causing gene and to understand the biological pathways leading to complement activation.
The eyes of monkey are structurally similar to human eyes which make them extremely valuable for macular degeneration studies. However, there are limitations in using this species over other laboratory animals. Monkeys have a relatively longer life span, have a longer gestation period, have a lower birth numbers resulting in a slower rate of expanding the pedigree, more difficult to genetically manipulate, and the cost of maintenance is significantly higher. In the other laboratory animals, the differences in the eye structure have been considered to be a disadvantage for using them as AMD models. However, they are easier to maintain and less expensive. This has made the development of a mouse model of AMD very attractive, and a number of mouse AMD models have been reported recently.
Chi Z-L, Yoshida T, Lambris JD, and Iwata T. Suppression of drusen formation by compstatin, a peptide inhibitor of complement C3 activation, on Cynomolgus monkey with early-onset macular degeneration.
Currrent Topics on Complement and Eye Disease, Advances in Experimental Medicine and Biology 703:127-135 (2010)
Umeda S, Suzuki MT , Okamoto H, Ono F, Mizota A, Terao K, Yoshikawa Y, Tanaka Y, and Iwata T. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (Macaca fascicularis).
FASEB Journal 19:1683-1685 (2005)
Umeda S, Ayyagari R, Allikmets R, Suzuki MT, Karoukis AJ, Ambasudhan R, Zernant J, Okamoto H, Ono F, Terao K, Atsushi M, Yoshikawa Y, Tanaka Y, and Iwata T. Early onset macular degeneration with drusen in a cynomolgus monkey (Macaca fascicularis) pedigree caused by a novel gene mutation.
Investive Ophthalmology and Visual Science 46:683-691 (2005)
Umeda S, Suzuki MT, Yoshikawa Y, Iwata F, Fujiki K, Kanai A, Sanuki N, Tanaka Y, and Iwata T. Cloning and Characterization of ELVLO4 Gene in Cynomolgus (Macaca fascicularis) Monkey.
Experimental Animal 52:(2) (2003)

