Glaucoma and Optic Neuropathy
Exploring the Genetic Landscape of Childhood Glaucoma
Childhood glaucoma, a significant cause of global blindness, represents a heterogeneous group of disorders categorized into primary or secondary forms. Primary childhood glaucoma stands as the most prevalent subtype, comprising primary congenital glaucoma (PCG) and juvenile open-angle glaucoma (JOAG). Presently, multiple genes are implicated in inherited forms of primary childhood glaucoma. This comprehensive review delves into genetic investigations into primary childhood glaucoma, with a focus on identifying causative genes, understanding their inheritance patterns, exploring essential biological pathways in disease pathogenesis, and utilizing animal models to study these mechanisms. Specifically, attention is directed towards genes such as CYP1B1 (cytochrome P450 family 1 subfamily B member 1), LTBP2 (latent transforming growth factor beta binding protein 2), TEK (TEK receptor tyrosine kinase), ANGPT1 (angiopoietin 1), and FOXC1 (forkhead box C1), all associated with PCG; and MYOC (myocilin), associated with JOAG. Through exploring these genetic factors, this review aims to deepen our understanding of the intricate pathogenesis of primary childhood glaucoma, thereby facilitating the development of enhanced diagnostic and therapeutic strategies.
Pan Y, Iwata T. Exploring the Genetic Landscape of Childhood Glaucoma. Children (Basel). 2024 Apr 9;11(4):454. doi: 10.3390/children11040454. PMID: 38671671
Molecular genetics of inherited normal tension glaucoma
Normal tension glaucoma (NTG) is a complex optic neuropathy characterized by progressive retinal ganglion cell death and glaucomatous visual field loss, despite normal intraocular pressure (IOP). This condition poses a unique clinical challenge due to the absence of elevated IOP, a major risk factor in typical glaucoma. Recent research indicates that up to 21% of NTG patients have a family history of glaucoma, suggesting a genetic predisposition. In this comprehensive review using PubMed studies from January 1990 to December 2023, our focus delves into the genetic basis of autosomal dominant NTG, the only known form of inheritance for glaucoma. Specifically exploring optineurin (OPTN), TANK binding kinase 1 (TBK1),
methyltransferase‑like 23 (METTL23), and myocilin (MYOC) mutations, we summarize their clinical manifestations, mutant protein behaviors, relevant animal models, and potential therapeutic pathways. This exploration aims to illuminate the intricate pathogenesis of NTG, unraveling the contribution of these genetic components to its complex development.
Pan Y, Iwata T. Molecular genetics of inherited normal tension glaucoma. Indian J Ophthalmol. 2024 Feb 23. doi: 10.4103/IJO.IJO_3204_23. Online ahead of print.PMID: 38389252
METTL23 mutation alters histone H3R17 methylation in normal-tension glaucoma
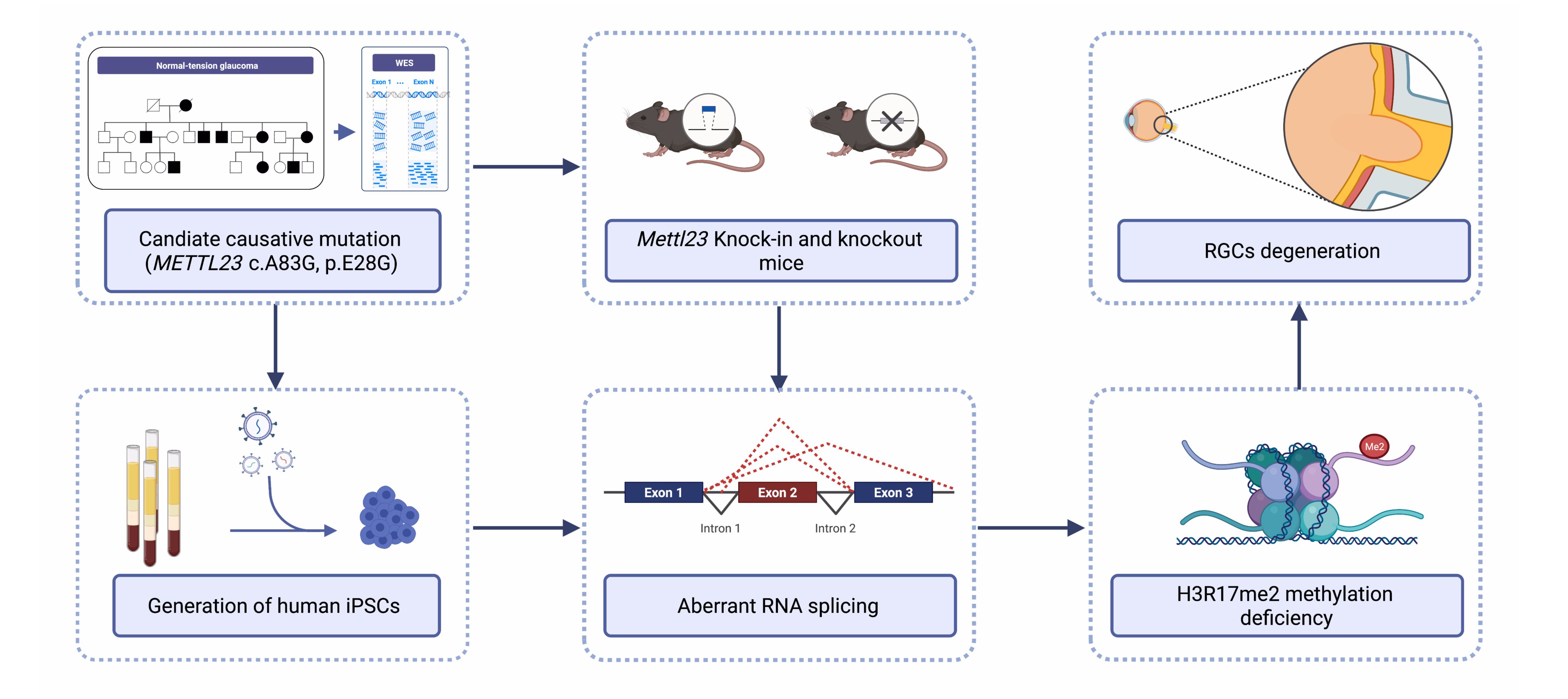 Normal-tension glaucoma (NTG) is a heterogeneous disease characterized by retinal ganglion cell (RGC) death leading to cupping of the optic nerve head and visual field loss at normal intraocular pressure (IOP). The pathogenesis of NTG remains unclear. Here, we described a single nucleotide mutation in exon 2 of the methyltransferase like 23 (METTL23) gene identified in a three-generation Japanese NTG family. This mutation caused METTL23 mRNA aberrant splicing, which abolished normal protein production and altered subcellular localization. Mettl23 knock-in (Mettl23+/G & Mettl23G/G) and knockout (Mettl23+/- & Mettl23-/-) mice developed a glaucoma phenotype without elevated IOP. METTL23 is a histone arginine methyltransferase expressed in murine and macaque RGCs. However, the novel mutation reduced Mettl23 expression in RGCs of Mettl23G/G mice, which recapitulated both clinical and biological phenotypes. Moreover, our findings demonstrated that Mettl23 catalyzed the dimethylation of H3R17 in the retina, and was required for the transcription of pS2, an estrogen receptor α target gene that was critical to RGC homeostasis through the negative regulation of NF-κB-mediated TNF-α/IL-1β feedback. These findings suggest an etiologic role of METTL23 in NTG with tissue-specific pathology.
Normal-tension glaucoma (NTG) is a heterogeneous disease characterized by retinal ganglion cell (RGC) death leading to cupping of the optic nerve head and visual field loss at normal intraocular pressure (IOP). The pathogenesis of NTG remains unclear. Here, we described a single nucleotide mutation in exon 2 of the methyltransferase like 23 (METTL23) gene identified in a three-generation Japanese NTG family. This mutation caused METTL23 mRNA aberrant splicing, which abolished normal protein production and altered subcellular localization. Mettl23 knock-in (Mettl23+/G & Mettl23G/G) and knockout (Mettl23+/- & Mettl23-/-) mice developed a glaucoma phenotype without elevated IOP. METTL23 is a histone arginine methyltransferase expressed in murine and macaque RGCs. However, the novel mutation reduced Mettl23 expression in RGCs of Mettl23G/G mice, which recapitulated both clinical and biological phenotypes. Moreover, our findings demonstrated that Mettl23 catalyzed the dimethylation of H3R17 in the retina, and was required for the transcription of pS2, an estrogen receptor α target gene that was critical to RGC homeostasis through the negative regulation of NF-κB-mediated TNF-α/IL-1β feedback. These findings suggest an etiologic role of METTL23 in NTG with tissue-specific pathology.
Pan Y, Suga A, Kimura I, Kimura C, Minegishi Y, Nakayama M, Yoshitake K, Iejima D, Minematsu N, Yamamoto M, Mabuchi F, Takamoto M, Shiga Y, Araie M, Kashiwagi K, Aihara M, Nakazawa T, Iwata T. J Clin Invest. 2022 Sep 13:e153589. doi: 10.1172/JCI153589. Online ahead of print.PMID: 36099048
Novel mutations in malonyl-CoA-acyl carrier protein transacylase provoke autosomal recessive optic neuropathy
 Inherited optic neuropathies are rare eye diseases of optic nerve dysfunction that present in various genetic forms. Previously, mutation in three genes encoding mitochondrial proteins has been implicated in autosomal recessive forms of optic atrophy that involve progressive degeneration of optic nerve and retinal ganglion cells (RGC). Using whole exome analysis, a novel double homozygous mutation p.L81R and pR212W in malonyl CoA-acyl carrier protein transacylase (MCAT), a mitochondrial protein involved in fatty acid biosynthesis, has now been identified as responsible for an autosomal recessive optic neuropathy from a Chinese consanguineous family. MCAT is expressed in RGC that are rich in mitochondria. The disease variants lead to structurally unstable MCAT protein with significantly reduced intracellular expression. RGC-specific knockdown of Mcat in mice, lead to an attenuated retinal neurofiber layer, that resembles the phenotype of optic neuropathy. These results indicated that MCAT plays an essential role in mitochondrial function and maintenance of RGC axons, while novel MCAT p.L81R and p.R212W mutations can lead to optic neuropathy.
Inherited optic neuropathies are rare eye diseases of optic nerve dysfunction that present in various genetic forms. Previously, mutation in three genes encoding mitochondrial proteins has been implicated in autosomal recessive forms of optic atrophy that involve progressive degeneration of optic nerve and retinal ganglion cells (RGC). Using whole exome analysis, a novel double homozygous mutation p.L81R and pR212W in malonyl CoA-acyl carrier protein transacylase (MCAT), a mitochondrial protein involved in fatty acid biosynthesis, has now been identified as responsible for an autosomal recessive optic neuropathy from a Chinese consanguineous family. MCAT is expressed in RGC that are rich in mitochondria. The disease variants lead to structurally unstable MCAT protein with significantly reduced intracellular expression. RGC-specific knockdown of Mcat in mice, lead to an attenuated retinal neurofiber layer, that resembles the phenotype of optic neuropathy. These results indicated that MCAT plays an essential role in mitochondrial function and maintenance of RGC axons, while novel MCAT p.L81R and p.R212W mutations can lead to optic neuropathy.
Li H, Yuan S, Minegishi Y, Suga A, Yoshitake K, Sheng X, Ye J, Smith S, Bunkoczi G, Yamamoto M, Iwata T. Novel mutations in malonyl-CoA-acyl carrier protein transacylase provoke autosomal recessive optic neuropathy. Hum Mol Genet. 2020 Jan 9. pii: ddz311. doi: 10.1093/hmg/ddz311. [Epub ahead of print] PubMed PMID: 31915829.
Optineurin E50K triggers BDNF deficiency-mediated mitochondrial dysfunction in retinal photoreceptor cell line
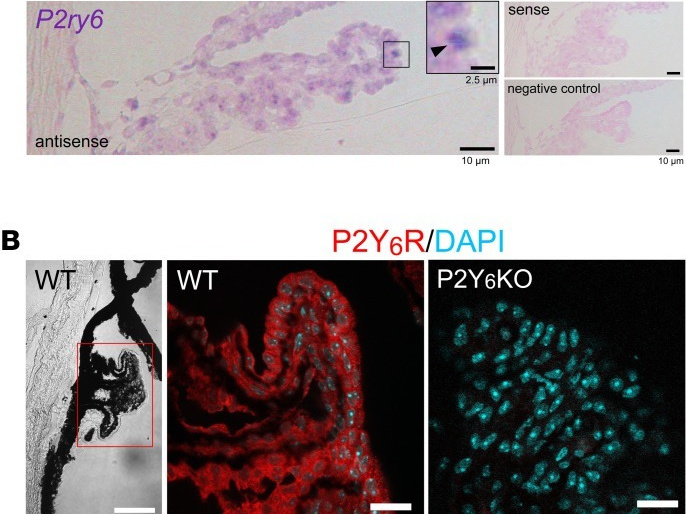
 IGlaucoma is an optic neuropathy characterized by progressive degeneration of retinal ganglion cells (RGCs) and visual loss. Although one of the highest risk factors for glaucoma is elevated intraocular pressure (IOP) and reduction in IOP is the only proven treatment, the mechanism of IOP regulation is poorly understood. We report that the P2Y6 receptor is critical for lowering IOP and that ablation of the P2Y6 gene in mice (P2Y6KO) results in hypertensive glaucoma-like optic neuropathy. Topically applied uridine diphosphate, an endogenous selective agonist for the P2Y6 receptor, decreases IOP. The P2Y6 receptor was expressed in nonpigmented epithelial cells of the ciliary body and controlled aqueous humor dynamics. P2Y6KO mice exhibited sustained elevation of IOP, age-dependent damage to the optic nerve, thinning of ganglion cell plus inner plexiform layers, and a reduction of RGC numbers. These changes in P2Y6KO mice were attenuated by an IOP lowering agent. Consistent with RGC damage, visual functions were impaired in middle-aged P2Y6KO mice. We also found that expression and function of P2Y6 receptors in WT mice were significantly reduced by aging, another important risk factor for glaucoma. In summary, our data show that dysfunctional purinergic signaling causes IOP dysregulation, resulting in glaucomatous optic neuropathy.
IGlaucoma is an optic neuropathy characterized by progressive degeneration of retinal ganglion cells (RGCs) and visual loss. Although one of the highest risk factors for glaucoma is elevated intraocular pressure (IOP) and reduction in IOP is the only proven treatment, the mechanism of IOP regulation is poorly understood. We report that the P2Y6 receptor is critical for lowering IOP and that ablation of the P2Y6 gene in mice (P2Y6KO) results in hypertensive glaucoma-like optic neuropathy. Topically applied uridine diphosphate, an endogenous selective agonist for the P2Y6 receptor, decreases IOP. The P2Y6 receptor was expressed in nonpigmented epithelial cells of the ciliary body and controlled aqueous humor dynamics. P2Y6KO mice exhibited sustained elevation of IOP, age-dependent damage to the optic nerve, thinning of ganglion cell plus inner plexiform layers, and a reduction of RGC numbers. These changes in P2Y6KO mice were attenuated by an IOP lowering agent. Consistent with RGC damage, visual functions were impaired in middle-aged P2Y6KO mice. We also found that expression and function of P2Y6 receptors in WT mice were significantly reduced by aging, another important risk factor for glaucoma. In summary, our data show that dysfunctional purinergic signaling causes IOP dysregulation, resulting in glaucomatous optic neuropathy.
Shinozaki Y, Kashiwagi K, Namekata K, Takeda A, Ohno N, Robaye B, Harada T, Iwata T, Koizumi S. JCI Insight. 2017 Oct 5;2(19). pii: 93456. doi: 10.1172/jci.insight.93456. [Epub ahead of print] PMID: 28978804
Purinergic dysregulation causes hypertensive glaucoma-like optic neuropathy
 To determine a chemical agent that can reduce the aggregation of optineurin (OPTN) in cells differentiated from induced pluripotent stem cells obtained from a patient with normal-tension glaucoma (NTG) caused by an E50K mutation in the OPTN gene (OPTNE50K-NTG). Retinal ganglion cells (RGCs) were created from induced pluripotent stem cells derived from a healthy individual (wild-type [WT]-iPSCs) and from a patient with NTG due to OPTNE50K (E50K-iPSCs) mutation. The death of the induced RGCs was evaluated by counting the number of TUNEL- and ATH5-positive cells. Axonal growth was determined by measuring the axonal length of TUJ1-positive cells. OPTN aggregation was assessed by measuring the OPTN-positive area by immunofluorescence and by Western blotting. Autophagic flux assay was investigated by determining the light chain 3 (LC3)B-II/LC3B-I ratio and p62 expression by Western blotting. The results showed OPTNE50K aggregation, activation of astrocytes, reduction in the number of RGCs, and enhancement of apoptotic cell death in the in vitro OPTNE50K model of NTG. Timolol was found to reduce the OPTNE50K-positive area and decreased the insoluble OPTNE50K, suggesting that it has the potential of reducing the OPTNE50K aggregation. Timolol also increased the ATH5-positive cells, decreased TUNEL-positive cells, increased the LC3B-II/LC3B-I ratio, and decreased the expression of p62. These findings suggest that timolol might enhance autophagic flux, leading to reduced OPTNE50K aggregation. Timolol should be considered a potential therapeutic agent specific to OPTNE50K-NTG because it can reduce the OPTNE50K aggregation in E50K-iPSCs-RGCs by enhancing autophagic flux and neuroprotective effects.
To determine a chemical agent that can reduce the aggregation of optineurin (OPTN) in cells differentiated from induced pluripotent stem cells obtained from a patient with normal-tension glaucoma (NTG) caused by an E50K mutation in the OPTN gene (OPTNE50K-NTG). Retinal ganglion cells (RGCs) were created from induced pluripotent stem cells derived from a healthy individual (wild-type [WT]-iPSCs) and from a patient with NTG due to OPTNE50K (E50K-iPSCs) mutation. The death of the induced RGCs was evaluated by counting the number of TUNEL- and ATH5-positive cells. Axonal growth was determined by measuring the axonal length of TUJ1-positive cells. OPTN aggregation was assessed by measuring the OPTN-positive area by immunofluorescence and by Western blotting. Autophagic flux assay was investigated by determining the light chain 3 (LC3)B-II/LC3B-I ratio and p62 expression by Western blotting. The results showed OPTNE50K aggregation, activation of astrocytes, reduction in the number of RGCs, and enhancement of apoptotic cell death in the in vitro OPTNE50K model of NTG. Timolol was found to reduce the OPTNE50K-positive area and decreased the insoluble OPTNE50K, suggesting that it has the potential of reducing the OPTNE50K aggregation. Timolol also increased the ATH5-positive cells, decreased TUNEL-positive cells, increased the LC3B-II/LC3B-I ratio, and decreased the expression of p62. These findings suggest that timolol might enhance autophagic flux, leading to reduced OPTNE50K aggregation. Timolol should be considered a potential therapeutic agent specific to OPTNE50K-NTG because it can reduce the OPTNE50K aggregation in E50K-iPSCs-RGCs by enhancing autophagic flux and neuroprotective effects.
Inagaki S, Kawase K, Funato M, Seki J, Kawase C, Ohuchi K, Kameyama T, Ando S, Sato A, Morozumi W, Nakamura S, Shimazawa M, Iejima D, Iwata T, Yamamoto T, Kaneko H, Hara H. Invest Ophthalmol Vis Sci. 2018 May 1;59(6):2293-2304. doi: 10.1167/iovs.17-22975. PMID: 29847634
Mitochondrial pathogenic mechanism and degradation in optineurin E50K mutation-mediated retinal ganglion cell degeneration
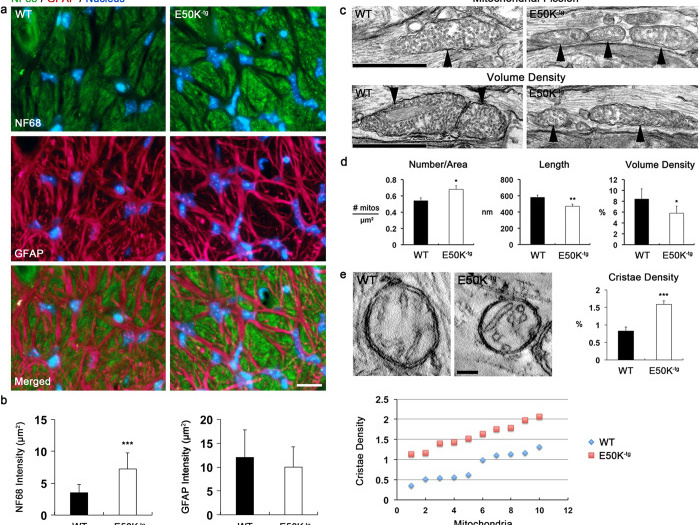
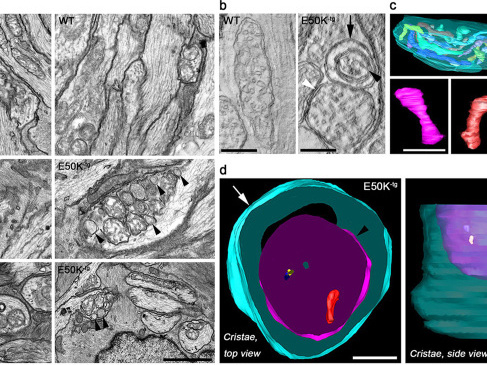 Mutations in optineurin (OPTN) are linked to the pathology of primary open angle glaucoma (POAG) and amyotrophic lateral sclerosis. Emerging evidence indicates that OPTN mutation is involved in accumulation of damaged mitochondria and defective mitophagy. Nevertheless, the role played by an OPTN E50K mutation in the pathogenic mitochondrial mechanism that underlies retinal ganglion cell (RGC) degeneration in POAG remains unknown. We show here that E50K expression induces mitochondrial fission-mediated mitochondrial degradation and mitophagy in the axons of the glial lamina of aged E50K-tg mice in vivo. While E50K activates the Bax pathway and oxidative stress, and triggers dynamics alteration-mediated mitochondrial degradation and mitophagy in RGC somas in vitro, it does not affect transport dynamics and fission of mitochondria in RGC axons in vitro. These results strongly suggest that E50K is associated with mitochondrial dysfunction in RGC degeneration in synergy with environmental factors such as aging and/or oxidative stress.
Mutations in optineurin (OPTN) are linked to the pathology of primary open angle glaucoma (POAG) and amyotrophic lateral sclerosis. Emerging evidence indicates that OPTN mutation is involved in accumulation of damaged mitochondria and defective mitophagy. Nevertheless, the role played by an OPTN E50K mutation in the pathogenic mitochondrial mechanism that underlies retinal ganglion cell (RGC) degeneration in POAG remains unknown. We show here that E50K expression induces mitochondrial fission-mediated mitochondrial degradation and mitophagy in the axons of the glial lamina of aged E50K-tg mice in vivo. While E50K activates the Bax pathway and oxidative stress, and triggers dynamics alteration-mediated mitochondrial degradation and mitophagy in RGC somas in vitro, it does not affect transport dynamics and fission of mitochondria in RGC axons in vitro. These results strongly suggest that E50K is associated with mitochondrial dysfunction in RGC degeneration in synergy with environmental factors such as aging and/or oxidative stress.
Shim MS, Takihara Y, Kim KY, Iwata T, Yue BY, Inatani M, Weinreb RN, Perkins GA, Ju WK. Sci Rep. 2016 Sep 22;6:33830. doi: 10.1038/srep33830. Erratum in: Sci Rep. 2017 Jan 19;7:40460. PMID: 27654856
Shim MS, Takihara Y, Kim KY, Iwata T, Yue BY, Inatani M, Weinreb RN, Perkins GA, Ju WK. Sci Rep. 2017 Jan 19;7:40460. doi: 10.1038/srep40460. No abstract available. PMID: 28102236
Significance of optineurin mutations in glaucoma and other diseases
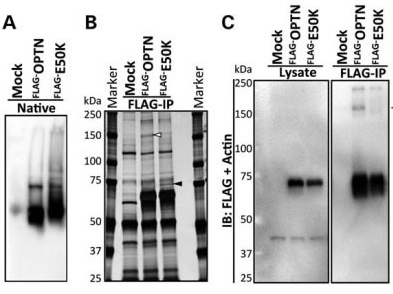 Glaucoma is one of the leading causes of bilateral blindness, affecting nearly 57 million people worldwide. Glaucoma is characterized by a progressive loss of retinal ganglion cells and is often associated with intraocular pressure (IOP). Normal tension glaucoma (NTG), marked by normal IOP but progressive glaucoma, is incompletely understood. In 2002, Sarfarazi et al. identified FIP-2 gene mutations responsible for hereditary NTG, renaming this gene "optineurin" (OPTN). Further investigations by multiple groups worldwide showed that OPTN is involved in several critical cellular functions, such as NF-κB regulation, autophagy, and vesicle transport. Recently, OPTN mutations were found to cause amyotrophic lateral sclerosis (ALS). Surprisingly, a mutation in the OPTN interacting protein, i.e., the duplication of TANK binding protein 1 (TBK1) gene, also can cause both NTG and ALS. These phenotypically distinct neuronal diseases are now merging into one common pathological mechanism by these two genes. TBK1 inhibition has emerged as a potential therapy for NTG. In this manuscript, we focus on the OPTN E50K mutation, the most common mutation for NTG, to describe the molecular mechanism of NTG by expressing a mutant Optn gene in cells and genetically modified mice. Patient iPS cells were developed and differentiated into neural cells to observe abnormal behavior and the impact of the E50K mutation. These in vitro studies were further extended to identify the inhibitors BX795 and amlexanox, which have the potential to reverse the disease-causing phenomenon in patient's neural cells. Here we show for the first time that amlexanox protects RGCs in Optn E50K knock-in mice.
Glaucoma is one of the leading causes of bilateral blindness, affecting nearly 57 million people worldwide. Glaucoma is characterized by a progressive loss of retinal ganglion cells and is often associated with intraocular pressure (IOP). Normal tension glaucoma (NTG), marked by normal IOP but progressive glaucoma, is incompletely understood. In 2002, Sarfarazi et al. identified FIP-2 gene mutations responsible for hereditary NTG, renaming this gene "optineurin" (OPTN). Further investigations by multiple groups worldwide showed that OPTN is involved in several critical cellular functions, such as NF-κB regulation, autophagy, and vesicle transport. Recently, OPTN mutations were found to cause amyotrophic lateral sclerosis (ALS). Surprisingly, a mutation in the OPTN interacting protein, i.e., the duplication of TANK binding protein 1 (TBK1) gene, also can cause both NTG and ALS. These phenotypically distinct neuronal diseases are now merging into one common pathological mechanism by these two genes. TBK1 inhibition has emerged as a potential therapy for NTG. In this manuscript, we focus on the OPTN E50K mutation, the most common mutation for NTG, to describe the molecular mechanism of NTG by expressing a mutant Optn gene in cells and genetically modified mice. Patient iPS cells were developed and differentiated into neural cells to observe abnormal behavior and the impact of the E50K mutation. These in vitro studies were further extended to identify the inhibitors BX795 and amlexanox, which have the potential to reverse the disease-causing phenomenon in patient's neural cells. Here we show for the first time that amlexanox protects RGCs in Optn E50K knock-in mice.
Minegishi Y, Nakayama M, Iejima D, Kawase K, Iwata T. Prog Retin Eye Res. 2016 Nov;55:149-181. doi: 0.1016/j.preteyeres.2016.08.002. Epub 2016 Sep 29. PMID: 27693724
Enhanced optineurin E50K-TBK1 interaction evokes protein insolubility and initiates familial primary open-angle glaucoma
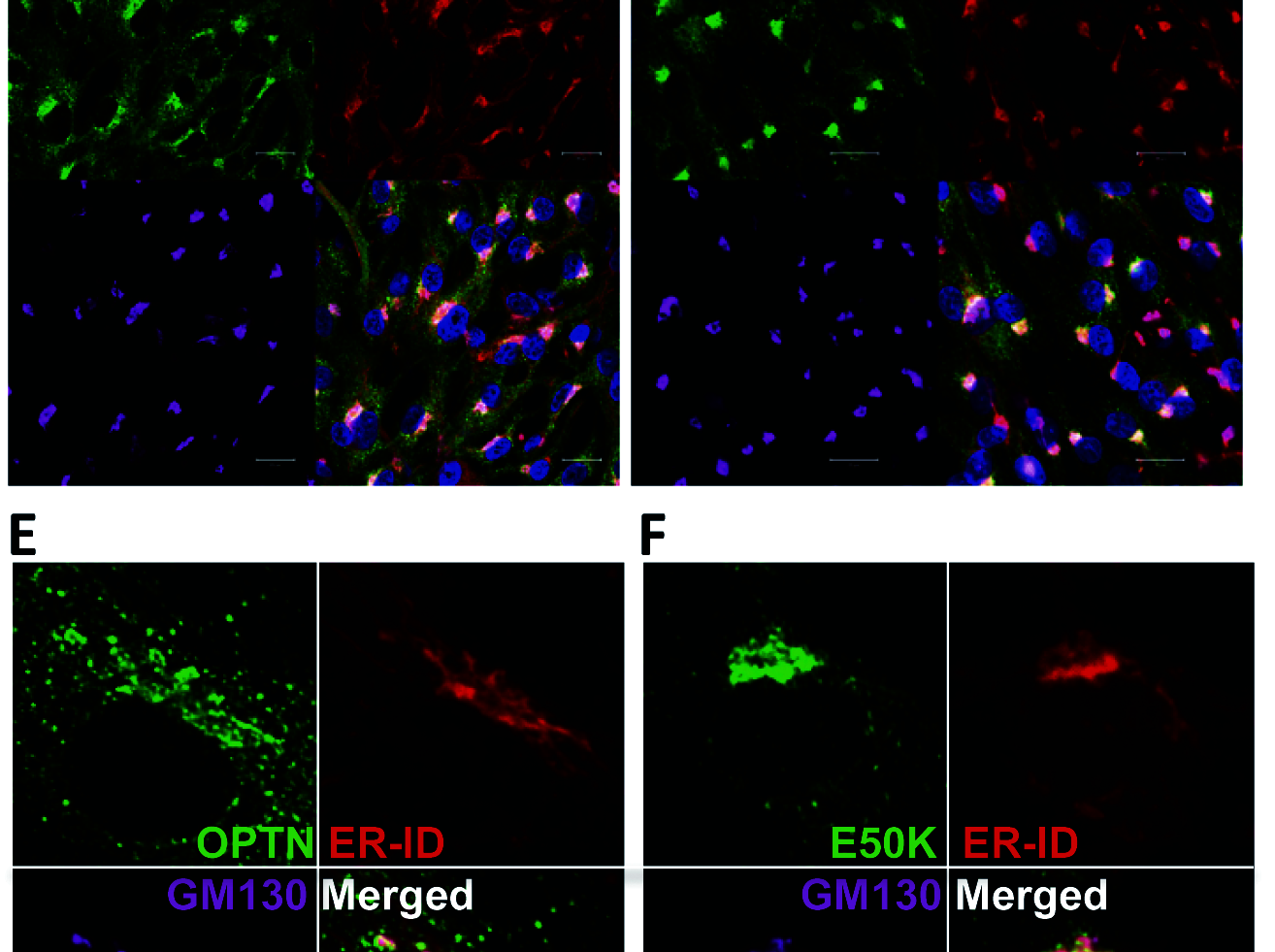 Glaucoma is the leading cause for blindness affecting 60 million people worldwide. The optineurin (OPTN) E50K mutation was first identified in familial primary open-angle glaucoma (POAG), the onset of which is not associated with intraocular pressure (IOP) elevation, and is classified as normal-tension glaucoma (NTG). Optineurin (OPTN) is a multifunctional protein and its mutations are associated with neurodegenerative diseases such as POAG and amyotrophic lateral sclerosis (ALS). We have previously described an E50K mutation-carrying transgenic (E50K-tg) mouse that exhibited glaucomatous phenotypes of decreased retinal ganglion cells (RGCs) and surrounding cell death at normal IOP. Further phenotypic analysis of these mice revealed persistent reactive gliosis and E50K mutant protein deposits in the outer plexiform layer (OPL). Over-expression of E50K in HEK293 cells indicated accumulation of insoluble OPTN in the endoplasmic
Glaucoma is the leading cause for blindness affecting 60 million people worldwide. The optineurin (OPTN) E50K mutation was first identified in familial primary open-angle glaucoma (POAG), the onset of which is not associated with intraocular pressure (IOP) elevation, and is classified as normal-tension glaucoma (NTG). Optineurin (OPTN) is a multifunctional protein and its mutations are associated with neurodegenerative diseases such as POAG and amyotrophic lateral sclerosis (ALS). We have previously described an E50K mutation-carrying transgenic (E50K-tg) mouse that exhibited glaucomatous phenotypes of decreased retinal ganglion cells (RGCs) and surrounding cell death at normal IOP. Further phenotypic analysis of these mice revealed persistent reactive gliosis and E50K mutant protein deposits in the outer plexiform layer (OPL). Over-expression of E50K in HEK293 cells indicated accumulation of insoluble OPTN in the endoplasmic
reticulum (ER). This phenomenon was consistent with the results seen in neurons derived from induced pluripotent stem cells (iPSCs) from E50K mutation-carrying NTG patients. The E50K mutant strongly interacted with TANK-binding kinase 1 (TBK1), which prohibited the proper oligomerization and solubility of OPTN, both of which are important for OPTN intracellular transition. Treatment with a TBK1
inhibitor, BX795, abrogated the aberrant insolubility of the E50K mutant. Here,we delineated the intracellular dynamics of the endogenous E50K mutant protein for the first time and demonstrated how this mutation causes OPTN insolubility,in association with TBK1, to evoke POAG.
Minegishi Y, Iejima D, Kobayashi H, Chi ZL, Kawase K, Yamamoto T, Seki T,Yuasa S, Fukuda K, Iwata T. Hum Mol Genet. 2013 Sep 1;22(17):3559-67. doi: 10.1093/hmg/ddt210. Epub 2013 May 12.
Characterization of hereditary normal tension glaucoma genes (OPTN, WDR36)
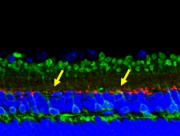 Glaucoma is a complex neurodegenerative disorders that is characterized by the irreversible loss of visual field by cell death of retinal ganglion cells, degeneration of axons in the optic nerve head known as glaucomatous cupping. Millions of people worldwide is affected as second leading cause of blindness. Glaucomas are categorized into primary and secondary types based on the etiology. Primary angle glaucomas are further classified as primary open glaucoma (POAG) and primary angle closure glaucoma (PACG). POAG is often, but not always, associated with elevated intraocular pressure (IOP), which is one of the main risk factors in glaucoma. However, about a third of all patients with POAG develop the disease without an IOP elevation, and in these patients, the IOP is continuously below 21 mmHg. This form of POAG is called normal tension glaucoma (NTG). A recent epidemiological study in Japan demonstrated that more than 90% of POAG cases were diagnosed as NTG. Majority of POAG are adult onset and less juvenile onset is observed. POAG is one of the major causes for irreversible blindness in about 12% globally.
Glaucoma is a complex neurodegenerative disorders that is characterized by the irreversible loss of visual field by cell death of retinal ganglion cells, degeneration of axons in the optic nerve head known as glaucomatous cupping. Millions of people worldwide is affected as second leading cause of blindness. Glaucomas are categorized into primary and secondary types based on the etiology. Primary angle glaucomas are further classified as primary open glaucoma (POAG) and primary angle closure glaucoma (PACG). POAG is often, but not always, associated with elevated intraocular pressure (IOP), which is one of the main risk factors in glaucoma. However, about a third of all patients with POAG develop the disease without an IOP elevation, and in these patients, the IOP is continuously below 21 mmHg. This form of POAG is called normal tension glaucoma (NTG). A recent epidemiological study in Japan demonstrated that more than 90% of POAG cases were diagnosed as NTG. Majority of POAG are adult onset and less juvenile onset is observed. POAG is one of the major causes for irreversible blindness in about 12% globally.
 Glaucoma is a genetically a complex eye disease, which attributed to several genes still unidentified. However, due to the progress of human genome project followed by the haplotype mapping project, number of microsatellite markers and single nucleotide polymorphisms has been identified. These polymorphic changes in the human genome are used to locate region associated with the disease. Large glaucoma families with Mendelian trait have been extensively studies for the past two decades by linkage analysis resulting in 25 chromosomal loci linked to POAG. Fifteen of these loci have been designated by the Human Genome Organization. Four of these genes myocilin, optineurin, WD repeat domain 36, and neurotrophin-4 have been identified. Mutations in these four genes exhibit high frequency of heterogeneity among different ethnic groups, and variable penetrance is often observed, making it difficult to understand whether the mutation is real. However, these problems are being solved not by genetics analysis but by the use of animal models generated by overexpressing mutant genes.
Glaucoma is a genetically a complex eye disease, which attributed to several genes still unidentified. However, due to the progress of human genome project followed by the haplotype mapping project, number of microsatellite markers and single nucleotide polymorphisms has been identified. These polymorphic changes in the human genome are used to locate region associated with the disease. Large glaucoma families with Mendelian trait have been extensively studies for the past two decades by linkage analysis resulting in 25 chromosomal loci linked to POAG. Fifteen of these loci have been designated by the Human Genome Organization. Four of these genes myocilin, optineurin, WD repeat domain 36, and neurotrophin-4 have been identified. Mutations in these four genes exhibit high frequency of heterogeneity among different ethnic groups, and variable penetrance is often observed, making it difficult to understand whether the mutation is real. However, these problems are being solved not by genetics analysis but by the use of animal models generated by overexpressing mutant genes.
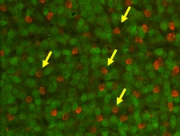
 The molecular pathways leading to the pathology of NTG and POAG are still unclear. We describe the phenotypic characteristics of transgenic mice over expressing wild-type or mutated optineurin (Optn). Mutations E50K, H486R, and optineurin with a deletion of the first (aa 153-174) or second (aa 426-461) leucine zipper were used for over expression. After 16 months, histological abnormalities were exclusively observed in the retina of E50K mutant mice with loss of retinal ganglion cells and connecting synapses in the peripheral retina leading to a thinning of the nerve fiber layer at the optic nerve head at normal IOP. E50K mice also showed massive apoptosis and degeneration of entire retina leading to approximately a 28% reduction of the retina thickness. At the molecular level, introduction of the E50K mutation disrupts the interaction between Optn and Rab8 GTPase, a protein involved in the regulation of vesicle transport from Golgi to plasma membrane. Wild-type Optn and an active GTP-bound form of Rab8 complex were localized at the Golgi complex. These data suggests that alternation of the Optn sequence can initiate significant retinal degeneration in mice.
The molecular pathways leading to the pathology of NTG and POAG are still unclear. We describe the phenotypic characteristics of transgenic mice over expressing wild-type or mutated optineurin (Optn). Mutations E50K, H486R, and optineurin with a deletion of the first (aa 153-174) or second (aa 426-461) leucine zipper were used for over expression. After 16 months, histological abnormalities were exclusively observed in the retina of E50K mutant mice with loss of retinal ganglion cells and connecting synapses in the peripheral retina leading to a thinning of the nerve fiber layer at the optic nerve head at normal IOP. E50K mice also showed massive apoptosis and degeneration of entire retina leading to approximately a 28% reduction of the retina thickness. At the molecular level, introduction of the E50K mutation disrupts the interaction between Optn and Rab8 GTPase, a protein involved in the regulation of vesicle transport from Golgi to plasma membrane. Wild-type Optn and an active GTP-bound form of Rab8 complex were localized at the Golgi complex. These data suggests that alternation of the Optn sequence can initiate significant retinal degeneration in mice.
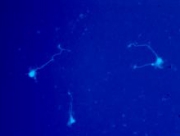
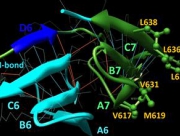 WD protein 36 (WDR36) has two domains with a similar fold. To address whether WDR36 is functionally important in the retina, we developed four transgenic mice strains overexpressing a wild-type Wdr36 and a mutant variants at (7th β-propeller of the second domain) equivalent to the location of the D658G mutation observed in POAG patients. A triple amino acid deletion of mouse Wdr36 at positions 605-607 corresponding to the deletion 657-659 in human developed progressive retinal degeneration at the peripheral retina at normal IOP. RGC and connecting amacrine cell synapses were affected at the peripheral retina. Axon outgrowth rate of cultured RGC directly isolated from transgenic animal was significantly reduced by the Wdr36 mutation compared with wild-type. Molecular modeling of wild and mutant mouse Wdr36 revealed that deletion 605-607 removed 3 residues and hydrogen bond, required to stabilize anti-parallel β-sheet of the 6th β-propeller in the second domain. We concluded that WDR36 plays important functional role in the retina homeostasis and mutation to this gene can cause devastating retinal damage. These data will improve understanding of the functional property of WDR36 in the retina and provide a new animal model for glaucoma therapeutics.
WD protein 36 (WDR36) has two domains with a similar fold. To address whether WDR36 is functionally important in the retina, we developed four transgenic mice strains overexpressing a wild-type Wdr36 and a mutant variants at (7th β-propeller of the second domain) equivalent to the location of the D658G mutation observed in POAG patients. A triple amino acid deletion of mouse Wdr36 at positions 605-607 corresponding to the deletion 657-659 in human developed progressive retinal degeneration at the peripheral retina at normal IOP. RGC and connecting amacrine cell synapses were affected at the peripheral retina. Axon outgrowth rate of cultured RGC directly isolated from transgenic animal was significantly reduced by the Wdr36 mutation compared with wild-type. Molecular modeling of wild and mutant mouse Wdr36 revealed that deletion 605-607 removed 3 residues and hydrogen bond, required to stabilize anti-parallel β-sheet of the 6th β-propeller in the second domain. We concluded that WDR36 plays important functional role in the retina homeostasis and mutation to this gene can cause devastating retinal damage. These data will improve understanding of the functional property of WDR36 in the retina and provide a new animal model for glaucoma therapeutics.
Chi Z-L, Yasumoto F, Sergeev Y, Minami M, Obazawa M, Kimura I, Takada Y, and Iwata T. Mutant WDR36 directly affects axon growth of retinal ganglion cells leading to progressive retinal degeneration in mice. Human Molecular Genetics 19:3806-3815 (2010)
Chi Z-L, Akahori, A, Obazawa M, Minami M, Noda T, Nakaya N, Tomarev S, Kawase K, Yamamoto T, Noda S, Sasaoka M, Shimazaki A, Takada Y, and Iwata T. Overexpression of optineurin E50K disrupts Rab8 interaction and leads to a progressive retinal degeneration in mice. Human Molecular Genetics 19:2605-2615 (2010)
Obazawa M, Mashima Y, Sanuki N, Noda S, Kudoh J, Shimizu N, Tanaka Y, and Iwata T. Comparable Analysis of Porcine Optineurin and Myocilin Expression in Trabecular Meshwork Cells and Astrocytes from Optic Nerve Head. Investigative Ophthalmology and Visual Science 45:2652-2659 (2004)
Izumi K, Mashima Y, Obazawa M, Ohtake Y, Tanino T, Miyata H, Tanaka Y, and Iwata T. Variants of Myocilin Gene in Japanese Patients With Normal Tension Glaucoma. Ophthalmic Research 35:345-350 (2003)

